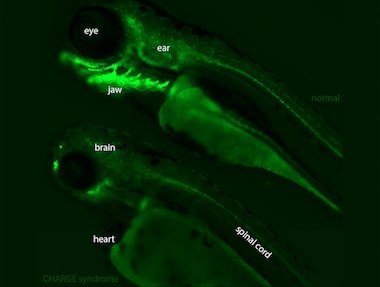
Perhaps the biggest question driving research in genetic diseases is how a change in DNA sequence affects an individual’s health. What are the intermediate steps from a mutation to disease manifestation? Chetana Sachidanandan and her team from the Institute for Genomics and Integrative Biology (IGIB), Delhi, set out to investigate this very question in CHARGE syndrome, a collection of diseases rooted in developmental errors.
CHARGE is an acronym for major defects in babies born with this condition: Coloboma (defect in formation of the eyes), Heart defect, Atresia choanae (blocking of one or both nasal passages by tissue overgrowth), Retarded growth & development, Genital abnormality and Ear abnormalities. Majority of clinical cases have mutations in a single gene, CHD7. The protein coded by this gene, also bearing the same name, influences transcription of a wide range of genes important in development. Using the zebrafish model, the research team uncovered the domino effect set in motion from mutations in the gene, and tested the effectiveness of RNA-based drugs to treat the syndrome. Mutations in the gene result in nonfunctional, shortened version of CHD7 protein. To mimic this condition and understand subsequent effects on embryo development, researchers turned down the expression of normal CHD7 in zebrafish.
Named for its zebra-like black and white stripes, zebrafish is a small freshwater fish endemic to Indian rivers. Several qualities about it make it an ideal model organism for studying genetic diseases: striking similarities between zebrafish and human genomes means experiments to figure out effects of genetic variations have direct relevance to human health. Plus, zebrafish embryos are transparent, making it possible to perform live imaging to monitor movement of cells in development. Embryos take only 2 – 3 days to grow into free swimming larvae, therefore unfolding of developmental defects can be easily observed.
Sequence and extent of gene expression has to be exquisitely specific for proper development. CHD7 is turned on very early on during development, starting from 2 – 4 cell stage embryo. This study shows that without this protein, zebrafish embryos exhibit defective differentiation and myelination of neurons of peripheral nervous system in addition to defects known in CHARGE patients . The research team also uncovered the role of a protein called sox10 in the progression of CHARGE syndrome. The number of cells making sox10 protein increases exponentially in the absence of CHD7 protein. To figure out if there was a cause-consequence relation between the two proteins, Zainab Asad (PhD student in Sachidanandan Lab and first author on this paper) decided to use RNA-based molecules to reduce the levels of sox10 in “mutant” zebrafish.
“To our surprise, we found that the extent of defects were visibly reduced in these conditions,” says Sachidanandan. Surprising, because a protein like CHD7 that is expressed so early, interacts with many, many proteins in the normal course of development; which of these to target to correct the course of disease in CHARGE, is not an easy question to answer. The odds can be worse than finding the proverbial needle in the haystack. “For this reason, I was skeptical about targeting sox10. It seemed a little too simplistic to think that reducing sox10 would help solve any of the problems in CHARGE. But Zainab was insistent. And the results took us all by surprise. This mechanism gives us yet another angle to pursue to develop a treatment,” says Sachidanandan.